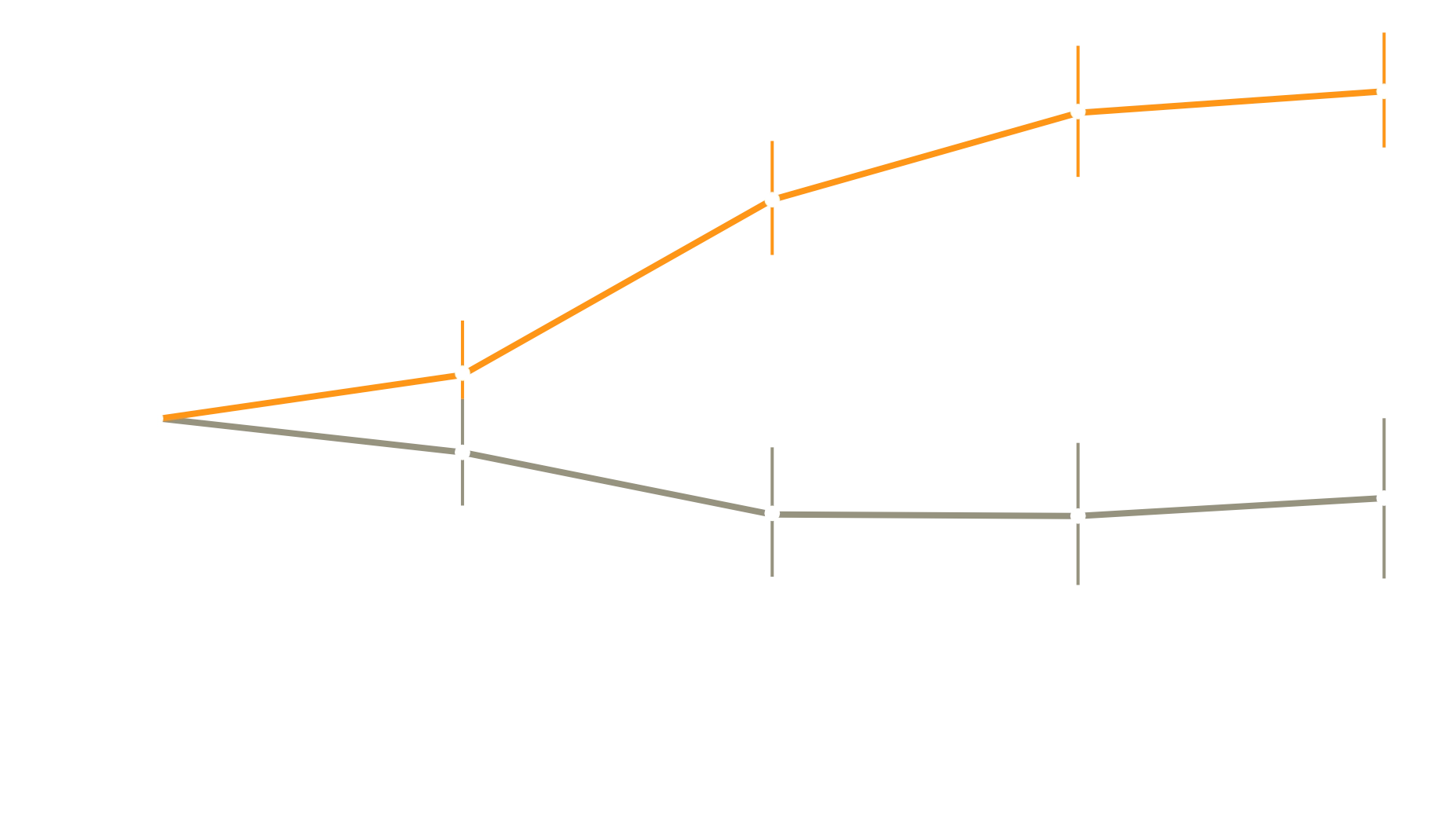
Mean (+/-SE) Change from Baseline in 6-Minute Walk Distance
Phase III, multicenter, double-blind, randomized, placebo-controlled, parallel-group trial
In people with PH-ILD, treatment with inhaled treprostinil improved exercise capacity and lowered the risk of clinical worsening
A 16-week study evaluating the safety and efficacy of inhaled treprostinil in patients with PH-ILD
Starting dose
Max. titration (every 3rd day)
Max. dose*
*Participants who reached 10–12 breaths at Week 16:
58%
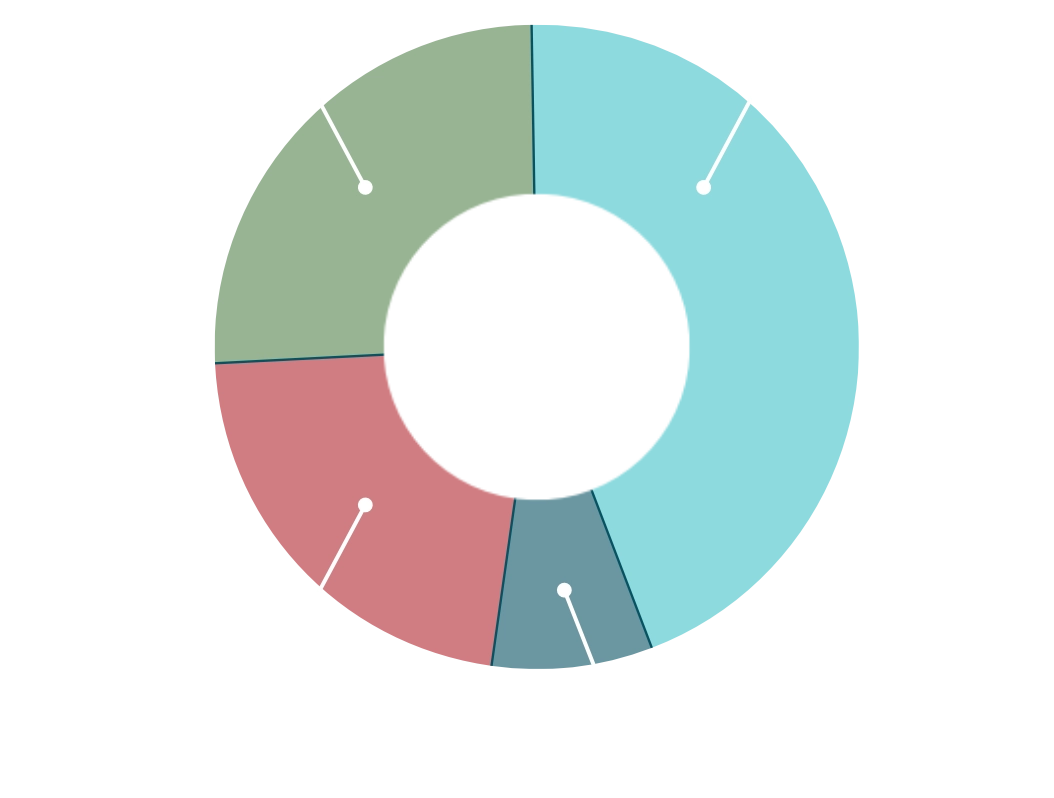
ILDs included those with idiopathic, environmental, autoimmune and occupational origins
Fewer patients in the treprostinil group had exacerbations of underlying lung disease
** Acute, clinically significant respiratory deterioration characterized by evidence of new widespread alveolar abnormality
The use of inhaled treprostinil was not associated with any decrement in lung function or oxygenation.
The safety profile of inhaled treprostinil was consistent with previous studies in people with pulmonary arterial hypertension (PAH).
| Variable | Inhaled treprostinil (n=163) | Placebo (n=163) |
|---|---|---|
| Most frequently occurring adverse events – no. of patients (%)‡ | ||
| Cough | 71 (44) | 54 (33) |
| Headache | 45 (28) | 32 (20) |
| Dyspnea | 41 (25) | 51 (31) |
| Dizziness | 30 (18) | 23 (14) |
| Nausea | 25 (15) | 26 (16) |
| Fatigue | 23 (14) | 23 (14) |
| Diarrhea | 22 (13) | 19 (12) |
| Throat irritation | 20 (12) | 6 (4) |
| Oropharyngeal pain | 18 (11) | 4 (3) |
| NT-proBNP increased | 9 (6) | 25 (15) |
| AEs leading to discontinuation | 16 (10) | 13 (8) |
‡ Shown are the most frequently occurring adverse events occurring in more than 10% of patients in either group in the safety population, which comprised all patients who underwent randomization and received at least one dose of treprostinil or placebo